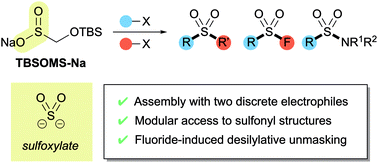
Silyloxymethanesulfinate as a sulfoxylate equivalent for the modular synthesis of sulfones and sulfonyl derivatives†
Abstract
An efficient protocol for the modular synthesis of sulfones and sulfonyl derivatives has been developed utilizing sodium tert-butyldimethylsilyloxymethanesulfinate (TBSOMS-Na) as a sulfoxylate (SO22−) equivalent. TBSOMS-Na, easily prepared from the commercial reagents Rongalite™ and TBSCl, serves as a potent nucleophile in S-alkylation and Cu-catalyzed S-arylation reactions with alkyl and aryl electrophiles. The sulfone products thus obtained can undergo the second bond formation at the sulfur center with various electrophiles without a separate unmasking step to afford sulfones and sulfonyl derivatives such as sulfonamides and sulfonyl fluorides.
Description
Indexed in scopushttps://www.scopus.com/authid/detail.uri?authorId=15076590100 |
Article metrics10.31763/DSJ.v5i1.1674 Abstract views : | PDF views : |
Cite |
Full Text Download Download
|
Conflict of interest
“Authors state no conflict of interest”
Funding Information
This research received no external funding or grants
Peer review:
Peer review under responsibility of Defence Science Journal
Ethics approval:
Not applicable.
Consent for publication:
Not applicable.
Acknowledgements:
None.