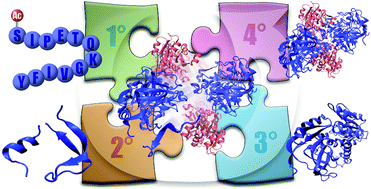
Higher-order structural characterisation of native proteins and complexes by top-down mass spectrometry
Abstract
In biology, it can be argued that if the genome contains the script for a cell's life cycle, then the proteome constitutes an ensemble cast of actors that brings these instructions to life. Their interactions with each other, co-factors, ligands, substrates, and so on, are key to understanding nearly any biological process. Mass spectrometry is well established as the method of choice to determine protein primary structure and location of post-translational modifications. In recent years, top-down fragmentation of intact proteins has been increasingly combined with ionisation of noncovalent assemblies under non-denaturing conditions, i.e., native mass spectrometry. Sequence, post-translational modifications, ligand/metal binding, protein folding, and complex stoichiometry can thus all be probed directly. Here, we review recent developments in this new and exciting field of research. While this work is written primarily from a mass spectrometry perspective, it is targeted to all bioanalytical scientists who are interested in applying these methods to their own biochemistry and chemical biology research.
Description
Indexed in scopushttps://www.scopus.com/authid/detail.uri?authorId=55194848800 |
Article metrics10.31763/DSJ.v5i1.1674 Abstract views : | PDF views : |
Cite |
Full Text Download Download
|
Conflict of interest
“Authors state no conflict of interest”
Funding Information
This research received no external funding or grants
Peer review:
Peer review under responsibility of Defence Science Journal
Ethics approval:
Not applicable.
Consent for publication:
Not applicable.
Acknowledgements:
None.