Total synthesis of nahuoic acid A via a putative biogenetic intramolecular Diels–Alder (IMDA) reaction†
Abstract
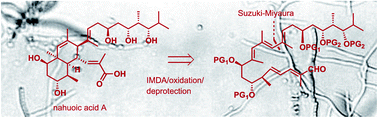
Inspired by the biogenetic proposal of an intramolecular Diels–Alder (IMDA) cycloaddition, the total synthesis of natural product nahuoic acid A, a cofactor-competitive inhibitor of the epigenetic enzyme lysine methyl transferase SETD8, has been carried out. A non-conjugated pentaenal precursor was synthesized with high levels of stereoselectivity at seven stereogenic centers and with the appropriate control of double bond geometries. Although the IMDA reaction of the non-conjugated pentaenal using Me2AlCl for catalysis at −40 °C selectively afforded the trans-fused diastereomer corresponding to the Re-endo mode of cycloaddition, under thermal reaction conditions it gave rise to a mixture of diastereomers, that preferentially formed through the exo mode, including the cis-fused angularly-methylated octahydronaphthalene diastereomer precursor of nahuoic acid A. The natural product could be obtained upon oxidation and overall deprotection of the hydroxyl groups present in the Si-exo IMDA diastereomer.
Description
Indexed in scopushttps://www.scopus.com/authid/detail.uri?authorId=57195714202 |
Article metrics10.31763/DSJ.v5i1.1674 Abstract views : | PDF views : |
Cite |
Full Text Download Download
|
Conflict of interest
“Authors state no conflict of interest”
Funding Information
This research received no external funding or grants
Peer review:
Peer review under responsibility of Defence Science Journal
Ethics approval:
Not applicable.
Consent for publication:
Not applicable.
Acknowledgements:
None.