Asymmetric hydrogenation for the synthesis of 2-substituted chiral morpholines†
Abstract
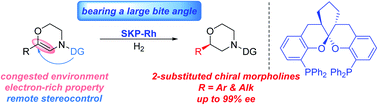
Asymmetric hydrogenation of unsaturated morpholines has been developed by using a bisphosphine-rhodium catalyst bearing a large bite angle. With this approach, a variety of 2-substituted chiral morpholines could be obtained in quantitative yields and with excellent enantioselectivities (up to 99% ee). The hydrogenated products could be transformed into key intermediates for bioactive compounds.
Description
Indexed in scopushttps://www.scopus.com/authid/detail.uri?authorId=57148846000 |
Article metrics10.31763/DSJ.v5i1.1674 Abstract views : | PDF views : |
Cite |
Full Text Download Download
|
Conflict of interest
“Authors state no conflict of interest”
Funding Information
This research received no external funding or grants
Peer review:
Peer review under responsibility of Defence Science Journal
Ethics approval:
Not applicable.
Consent for publication:
Not applicable.
Acknowledgements:
None.