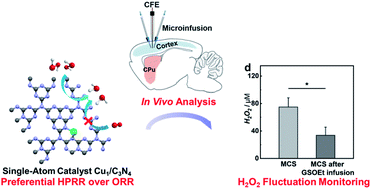
A single-atom Cu–N2 catalyst eliminates oxygen interference for electrochemical sensing of hydrogen peroxide in a living animal brain†
Abstract
Hydrogen peroxide (H2O2) plays essential roles in various physiological and pathological processes. The electrochemical hydrogen peroxide reduction reaction (HPRR) has been recognized as an efficient approach to H2O2 sensing; however, the HPRR has always suffered from low tolerance against the oxygen reduction reaction (ORR), resulting in poor selectivity of the HPRR-based sensing platform. In this study, we find that the electrochemical HPRR occurs preferentially compared to the ORR when isolated Cu atoms anchored on carbon nitride (Cu1/C3N4) are used as a single-atom electrocatalyst, which is theoretically attributed to the lower energy barrier of the HPRR than that of the ORR on a Cu1/C3N4 single-atom catalyst (SAC). With the Cu1/C3N4 SAC as the electrocatalyst, we fabricated microsensors that have a good response to H2O2, but not to O2 or other electroactive neurochemicals. When implanted into a living rat brain, the microsensor shows excellent in vivo sensing performance, enabling its application in real-time quantitative investigation of the dynamics of H2O2 production induced by mercaptosuccinate and glutathione monoethyl ester in a living animal brain.
Description
Indexed in scopushttps://www.scopus.com/authid/detail.uri?authorId=56984783000 |
Article metrics10.31763/DSJ.v5i1.1674 Abstract views : | PDF views : |
Cite |
Full Text Download Download
|
Conflict of interest
“Authors state no conflict of interest”
Funding Information
This research received no external funding or grants
Peer review:
Peer review under responsibility of Defence Science Journal
Ethics approval:
Not applicable.
Consent for publication:
Not applicable.
Acknowledgements:
None.