Rationalizing the generation of broad spectrum antibiotics with the addition of a positive charge†
Abstract
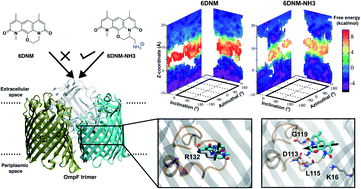
Antibiotic resistance of Gram-negative bacteria is largely attributed to the low permeability of their outer membrane (OM). Recently, we disclosed the eNTRy rules, a key lesson of which is that the introduction of a primary amine enhances OM permeation in certain contexts. To understand the molecular basis for this finding, we perform an extensive set of molecular dynamics (MD) simulations and free energy calculations comparing the permeation of aminated and amine-free antibiotic derivatives through the most abundant OM porin of E. coli, OmpF. To improve sampling of conformationally flexible drugs in MD simulations, we developed a novel, Monte Carlo and graph theory based algorithm to probe more efficiently the rotational and translational degrees of freedom visited during the permeation of the antibiotic molecule through OmpF. The resulting pathways were then used for free-energy calculations, revealing a lower barrier against the permeation of the aminated compound, substantiating its greater OM permeability. Further analysis revealed that the amine facilitates permeation by enabling the antibiotic to align its dipole to the luminal electric field of the porin and form favorable electrostatic interactions with specific, highly-conserved charged residues. The importance of these interactions in permeation was further validated with experimental mutagenesis and whole cell accumulation assays. Overall, this study provides insights on the importance of the primary amine for antibiotic permeation into Gram-negative pathogens that could help the design of future antibiotics. We also offer a new computational approach for calculating free-energy of processes where relevant molecular conformations cannot be efficiently captured.
Description
Indexed in scopushttps://www.scopus.com/authid/detail.uri?authorId=57140737800 |
Article metrics10.31763/DSJ.v5i1.1674 Abstract views : | PDF views : |
Cite |
Full Text Download Download
|
Conflict of interest
“Authors state no conflict of interest”
Funding Information
This research received no external funding or grants
Peer review:
Peer review under responsibility of Defence Science Journal
Ethics approval:
Not applicable.
Consent for publication:
Not applicable.
Acknowledgements:
None.